Post Bariatric Hypoglycemia (PBH)
PBH is an increasingly recognized chronic side effect of bariatric surgeries that are commonly performed as a treatment for obesity and related comorbidities. Neuroglycopenic symptoms can include shakiness, dizziness, confusion, sweating and loss of consciousness. These symptoms can be debilitating with a significant negative impact on quality of life, can dangerously impair normal day-to-day activities and can be life-threatening.
Bariatric surgery has proven to be the most effective treatment for severe obesity, leading to significant improvements in body mass index and obesity-related co-morbidities. It is estimated that over 9% of the U.S. population has a body mass index above 40 which is considered severely obese. With over 250,000 bariatric surgery procedures performed per year in the United States, postoperative prevalence of hypoglycemia symptoms in bariatric surgery patients is thought to be as high as 38.5%. There are currently no therapeutics approved by the FDA for the treatment of PBH.
Mizagliflozin is an investigational first-in-class, oral, small molecule drug candidate that reduces postprandial glucose absorption, secretion of insulin, and secretion of gastric inhibitory peptide, also known as glucose-dependent insulinotropic peptide (GIP). The molecule is being developed by Vogenx for the treatment of PBH and gastroparesis, both debilitating diseases with high unmet medical need in underserved patient populations. Mizagliflozin has been administered to over 500 subjects in clinical studies and has shown statistically significant reductions in postprandial glucose absorption as well as secretion of insulin and GIP.
Clinical Study VGX-001-011
This phase 2 clinical study (NCT05541939) was a multi-center, randomized, sequential crossover, single ascending dose study evaluating mizagliflozin in patients who suffer from post-bariatric hypoglycemia (PBH), a debilitating complication of bariatric surgery leading to recurrent postprandial hypoglycemia with neuroglycopenia. The study examined four doses of mizagliflozin in patients randomly assigned to one of two treatment arms. Safety, tolerability and pharmacodynamic response to mizagliflozin were assessed during a mixed meal tolerance test (MMTT).
Nine patients each received a baseline MMTT and were randomized to one of two treatment arms. Treatment Arm A received a 2.5 and 5.0 mg mizagliflozin capsule at sequential visits. Treatment Arm B received a 2.5 mg mizagliflozin liquid formulation and 10.0 mg mizagliflozin capsule at sequential visits. Mizagliflozin doses were administered 20 minutes prior to MMTT administration. Pharmacodynamic samples were taken at various times from 0 180 minutes, and glucose and insulin profiles determined.
The primary endpoints were safety and change in glucose nadir from baseline. Secondary endpoints included change from baseline peak plasma glucose and peak insulin. Levels of glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP) were examined as exploratory endpoints in a subset of patients.
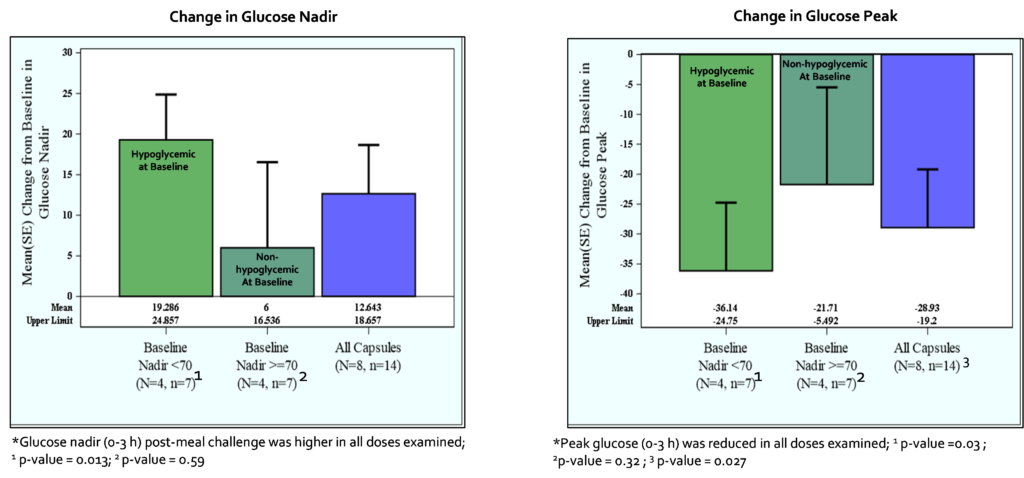
In patients experiencing hypoglycemia at baseline with a glucose nadir <70 mg/dL, the change from baseline mean glucose nadir for all capsule doses was 19.3 ± 14.7 mg/dL (p = 0.013); patients with glucose nadir at baseline ≥70 mg/dL showed a change from baseline mean glucose nadir for all capsule doses of 6.0 ± 27.9 mg/dL (p = 0.59). These data suggest mizagliflozin is effective in preventing hypoglycemic events without significantly impacting blood glucose levels in patients not experiencing hypoglycemia.
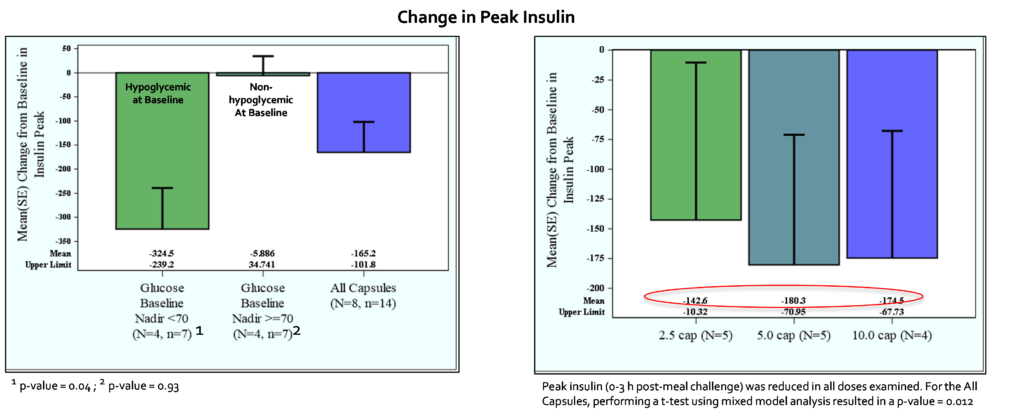
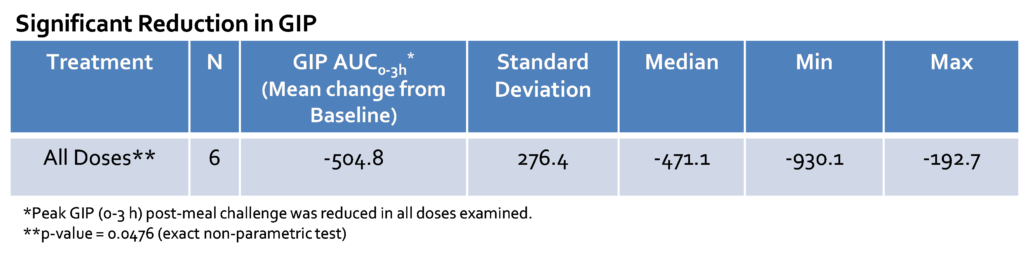
In all subjects, the mean glucose nadir change from baseline for all capsule doses was 12.6 ± 22.5 mg/dL (p = 0.045). The mean peak glucose change from baseline for all capsules was -29 ± 36.4 mg/dL (p = 0.027). The mean peak insulin change from baseline for all capsules was -165 ± 237 uU/ml (p = 0.012). In the subset analysis, mizagliflozin showed no significant effect on circulating GLP-1. In contrast, all mizagliflozin treatments in the subset analysis showed decreased peak GIP levels and AUC0-3h compared to baseline.
Mizagliflozin was well-tolerated. There were no treatment-related serious adverse events and no participant withdrawals. Adverse events were mild with the most common being nausea and abdominal bloating.
Clinical Study VGX-001-012
The multicenter, randomized, single-blind, placebo-controlled, dose-ranging and regimen-finding study enrolled 15 patients actively struggling with symptoms of PBH. Each patient was dosed with two doses of mizagliflozin and placebo, each for seven days (21-day study period) with a one-week washout period between regimens. Mixed meal tolerance tests were administered at the conclusion of each treatment period, and all patients wore blinded continuous glucose monitors throughout each treatment period. The primary endpoints were safety and change in glucose nadir from placebo. Secondary endpoints included change from placebo peak plasma glucose and peak insulin.
In patients experiencing hypoglycemia (<70 mg/dL) on placebo, the change from placebo mean glucose nadir for the 5.0 and 10.0 mg doses combined (the highest doses examined) was 18.0 ± 7.75 mg/dL (p = 0.028; n=9); patients with placebo glucose nadir ≥70 mg/dL showed a change from placebo mean glucose nadir for the 5.0 and 10.0 mg doses of -7.97 ± 6.22 mg/dL (p = 0.24; n=9). These data suggest mizagliflozin is effective in preventing hypoglycemic events without significantly impacting blood glucose levels in patients not experiencing hypoglycemia.
The mean peak glucose changes from placebo for the 5.0 and 10.0 mg doses was 57.6 ± 47.2 mg/dL (p = 0.004; n=9). The mean peak insulin change from placebo for the 5.0 and 10.0 mg doses was -156.5 ± -89.5 uU/ml (p = 0.074; n=9). In the exploratory analysis, mizagliflozin showed significant reductions of Level 3 hypoglycemia, 0.27 (p=0.023) and -0.29 (p=0.07) events/day versus placebo for the 5 and 10 mg dose, respectively. Combined 5 and 10 mg showed a reduction of 0.26 (p=0.003) events/day versus placebo. Additionally, 5 and 10 mg doses exhibited a 30.3% and 75.5% reduction in Level 2 hypoglycemic events from placebo as measured by blinded CGM.

EMERGE Phase 2b Clinical Study
Vogenx is currently preparing for initiation of the phase 2b EMERGE study evaluating mizagliflozin in PBH patients. This study is expected to initiate in 2025. Patients and investigators interested to learn more about this and other upcoming studies should contact the Vogenx clinical team here.
